
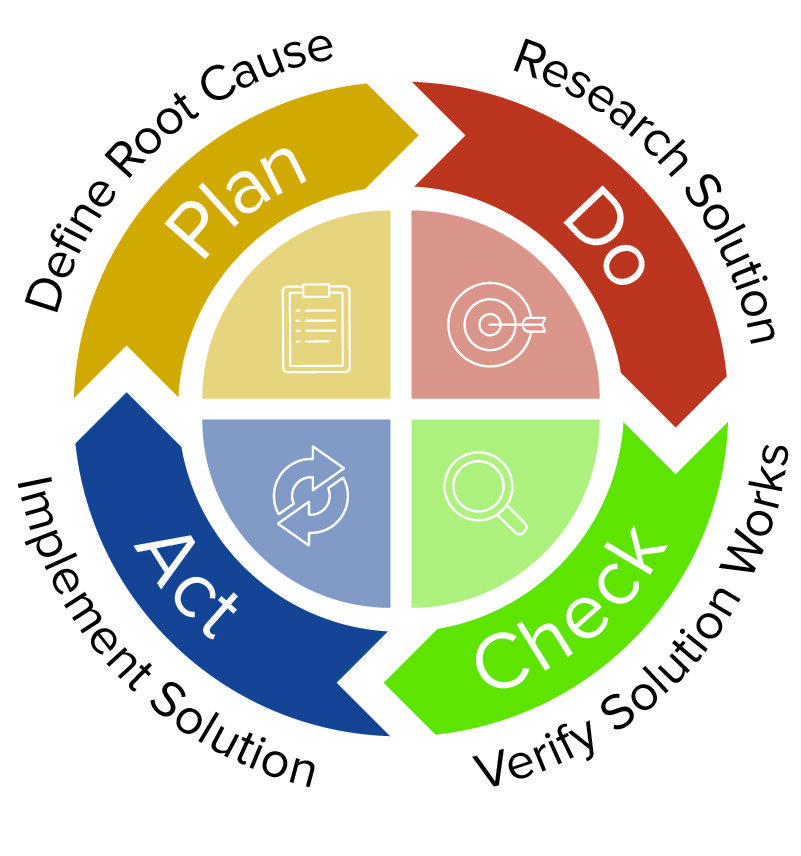
Here, tools such as a Gemba Walk Checklist can help companies find the cause of the problem rather than simply identifying the symptoms. By documenting what’s happening, where, when, and why, companies lay the groundwork for targeted follow-up actions that address root causes rather than symptoms. The first step in an effective CAPA assessment is pinpointing problems and describing them in detail. To make the most of this quality management framework, start with six fundamental CAPA process steps: 1) Pinpointing Key Problems
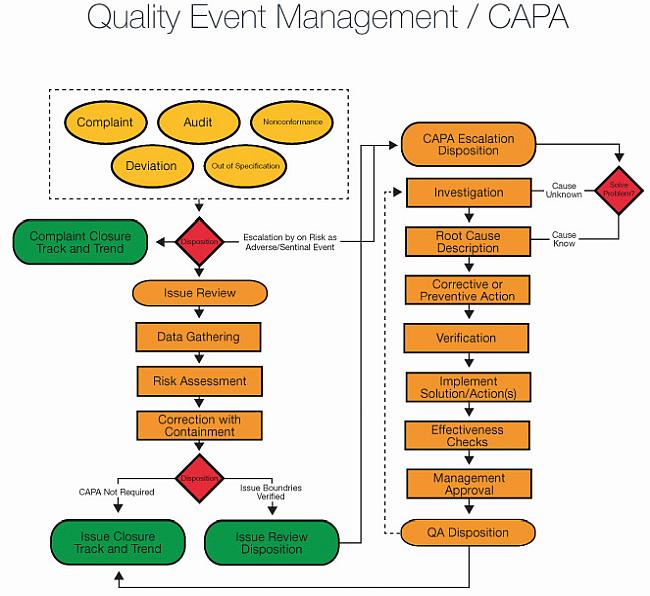
As a result, the corrective action and preventive action model offered by CAPA is a critical component of QMS frameworks because it helps organizations go beyond documentation to take action that improves the overall quality and reduces the risk of non-conformance. The CAPA system helps companies identify non-conformity issues that negatively impact quality, correct these issues through targeted action, and prevent these issues from recurring. Quality management systems (QMS) document all processes and policies required to achieve reliable quality levels in both staff operations and product outputs. Why do CAPA Requirements Matter for Quality Management Systems? These actions allow companies to eliminate potential problems prior to their occurrence, in turn saving both time and money. These actions go a step further than corrections to provide longer-term solutions.įinally, preventative actions are taken to eliminate the cause of potential non-conformities identified during a CAPA audit. CAPA stands for Corrective and Preventative Action (CAPA) and is an assessment tool designed to help companies identify and remediate operational, environmental, as well as safety issues by taking corrective and preventive action.Īccording to the FDA, CAPA management processes have three key components:Ĭorrections are actions taken to eliminate a specific non-conformity, such as defects in a product when it comes off production lines.Ĭorrective actions are taken to eliminate the cause of a problem or non-conformity and prevent a recurrence.


 0 kommentar(er)
0 kommentar(er)
